Is Your Contact Cleaner Compatible with Today’s Complex Electronics?
Some cleaners are speedy and effective but can attack plastic components.
With modern electronics getting smaller and more complex, finding a reliable cleaning method that is both effective and compatible with the different materials of electrical equipment can be difficult. The smallest contaminant can form a barrier between contacts and parts, or initiate dendritic growth, affecting the device’s efficiency and performance. This is why cleaning plays an important role in guaranteeing the quality of electronics equipment coming off the production line.
Contact cleaners are widely used in the industry due to ease of use and convenience. They clear particulate and oil residues from hard-to-reach places and refresh electrical connectivity on switches, relays, potentiometers and other devices. They safely rinse grit from hot motors and dust from inside electromechanical relays and keyboards. They are also effective in removing contaminants from hard-to-reach areas on connectors, cable harnesses, tuners, power supplies, encoders, distribution panels, junction boxes and switching devices.
The perfect contact cleaner should be nonflammable, noncorrosive and have strong dielectric properties. Ideally, it could even be sprayed on energized electrical circuits without concern. The cleaning agent must be safe for use on all component materials without risk of damaging delicate parts. These features may be the ultimate contact cleaning combination, but some electronics manufacturers may not be taking time to check the cleaners used are up to the job and compatible with electronic components.
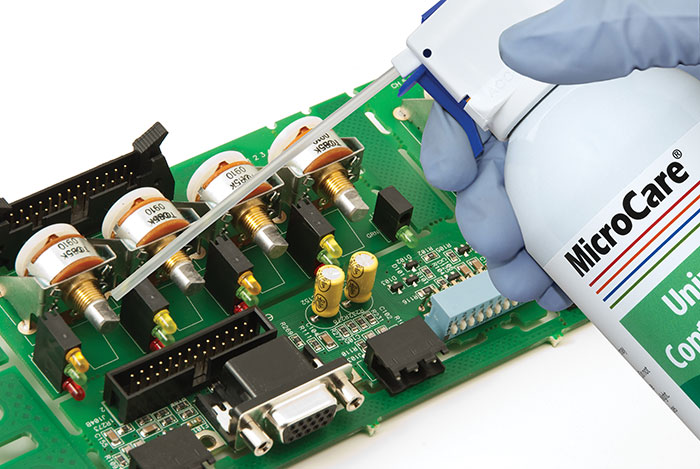
Figure 1. Contact cleaners flush away dust and lint from electronics equipment.
First step. Although the capability of the contact cleaner is important, so is its compatibility with the substrate being cleaned. When it comes to choosing the correct contact cleaner, first check for material compatibility. This is a critical area to investigate before any cleaning process is undertaken. Does the device consist of just one type of material, or is it made up of several materials with vulnerable components? Perhaps it has an LCD display made from transparent polycarbonate, contains inks, or includes rubber parts that may be damaged by aggressive solvents.
Think about what the electronics are made from. Many use a range of materials for their structure. One electronic component can be fiberglass, while others can contain materials like copper, elastomers or screen-printed parts. If unsure about the materials of construction, test before widespread deployment of a cleaner.
Contact cleaners. A strong cleaning fluid is often used to ensure cleanliness. It delivers excellent results and cleans quickly. However, a cleaner that is too strong can damage soft plastics, rubber, conformal coatings and even remove inks. It may appear to be a quick and effective solution to cleaning, but the unseen damage caused can be destructive to the component materials.
Modern contact cleaners can be an effective and safe method of cleaning. They remove oxides and other unwanted contaminants from the conductive surfaces of switches, connectors and other electrical components with surface contacts. They are particularly useful if cleaning is required for assemblies with varying material use or those where component parts have not yet been established. Strong cleaners may dissolve, craze or attack softer substrates; therefore, a milder plastic-safe cleaner is often the preferred choice. Ensure the contact cleaner is nonconductive, fast-drying, nonflammable, and safe on all materials of construction.
An array of contact cleaners is on the market. In the past, some technicians used IPA (isopropyl alcohol) with some success for contact cleaning. IPA is slow-drying and highly flammable, causing potential safety risks. In addition, oxides are typically not cleaned by IPA, so alcohol may not be the best choice. On the other hand, most modern contact cleaners, with their high volatility, dry quickly and without residue. They are also nonflammable and can be used on energized equipment.
Keep the Kb value low. The cleaning strength of a contact cleaner is frequently measured by an industry benchmark called the Kauri Butanol (Kb) value. If an electronic device is made from a variety of materials and includes plastic components, use a contact cleaner with a low Kb value. Stronger cleaning fluids with a high Kb value may have compatibility problems with soft plastics, coatings, inks and other components. Ideally, if delicate materials are within the device that requires cleaning, a Kb value of 15 to 40 is usually safest. This Kb range indicates the contact cleaner is mild and suitable for most surfaces.
Kb values can be found on the contact cleaner’s technical data sheet. The figure shown will help gauge the cleaner’s strength and suitability for the contaminant requiring removal. Cleaners with lower Kb values will remove greases but may not handle ionics and fluxes. Cleaners with higher Kb values may be speedy and effective but may attack plastic components. For this reason, consider the components to be cleaned and the materials of construction. Depending on the contamination, make sure the contact cleaner is strong enough to remove the contamination and clean effectively, while not affecting the components themselves.
A good method of ensuring a contact cleaner is working effectively without affecting the component material is to conduct a “cleaning trial” on a sacrificial or test part. The best practice is to start with a milder cleaner first and progressively try stronger cleaners until the optimal cleaning result is achieved. It is recommended tests be performed in more than one area on the part to ensure it is safe for all the materials the cleaner may contact, directly or indirectly.
Leading suppliers of cleaning solutions have field engineers who can provide guidance on testing cleaners and how to select the best one for the component and contamination. Often, the results are unexpected: some mild cleaners may clean as well as or even better than those with much higher Kb values, with the added benefit of maintaining material compatibility.
Many companies will conduct their own in-house cleaning trials, but in some instances, companies may send their sacrificial test parts to the cleaning fluid manufacturer for an in-lab cleaning assessment. Cleaning experiments are conducted on their parts and particular contamination to ensure effective cleaning with the fewest risk to the parts. The lab will typically present the client with a written report, including detailed recommendations on the best cleaner and cleaning methods to ensure cleanliness and safety.
What is out there? Many contact cleaners on the market combine a number of important features. They clean effectively, are worker safe, environmentally friendly and inexpensive. Although mild, they clean thoroughly and can rinse and flush away mineral and silicone oils, as well as remove dust, lint, grit and other particulates, making electronics components clean and ready for the next stage in the manufacturing process.
When choosing a contact cleaner, check its credentials. Not only does it need to clean well, but it must work with the components it is used on. It must be compatible with all materials of the assembly. Look at its Kb value: is it low enough to be suitable for all surfaces? Is it nonflammable, and can it safely clean a variety of electronic mechanisms from connectors and relays to wiring harnesses and mechanical devices, all while the equipment is energized and operating? Does it have strong dielectric properties to prevent electrical shorting while the cleaner is drying? Consider worker safety and environmental and regulatory compliance. Modern contact cleaners on the market today can comply with strict air quality regulations and are formulated to meet stringent safety requirements.
Do not overlook price point. The cleaner must work within the budget. Perhaps consider the use of a controlled dispensing system that attaches to the contact cleaner. This method delivers faster and better cleaning with less waste.
is a chemist at MicroCare Corp. (microcare.com) and holds a master’s in chemistry from Tufts University; lab@microcare.com.
Press Releases
- Hon Hai Technology Group (Foxconn) Commits To New 5-Year Sustainability Roadmap Through 2030
- Amtech Electrocircuits Navigates Supreme Court Tariff Ruling
- Escatec Appoints Christa Schnider as Chief Sales Officer to Drive Global Growth
- PulseForge Makes Major Breakthrough Which Allows Flux-less Soldering in an Ambient Environment







