2005 Articles
Localized Contamination, Big Problems
When it comes to some residues, ROSE testing is not so sweet.
Assemblers continue to overlook localized areas of contamination that are capable of causing product failures. By neglecting to examine sensitive localized areas, opportunities for electrochemical failures become exponential. This month’s case involves a customer seeing visible white residues and dendritic growth in a connector area. Luckily for them, the residues were visible, which led them to investigate their source and potential damage. (Such residues are often invisible and can go unnoticed until field problems occur.)
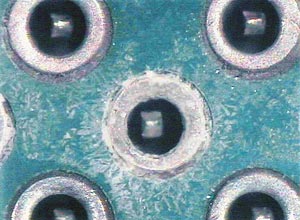 |
| Localized white residue on assembly. |
One assembly sample from a plant in Malaysia was sent to our laboratory for analysis. We used FTIR, SEM/EDX and ion chromatography analysis using localized extraction techniques. FTIR analysis showed only flux residues. SEM/EDX showed a concentration of tin, lead, oxygen and copper. The high concentration of tin and lead caused us to deduce that electromigration was occurring in this area, creating the white residue presence and visible dendritic growth (see figure). Because of these findings, we decided to analyze the area of white residue using ion chromatography in order to better understand what contaminants were present and what they indicated about the manufacturer’s process.
Using a C3 localized cleanliness tester, we extracted a residue sample from a 0.1 in2 area on the connector area and its casing, and a reference area on the connector area and casing. The tester showed “dirty” readings for the connector area, with white residue presence and its casing area (Table 1). Examining the reference areas on the connector and casing, we received “clean” readings for both. We took these four extracted samples and performed IC analysis. We found high levels of chloride, sulfate and weak organic acid (WOA) flux residues on the visible white residue sample from the connector and its casing. Conversely, we detected low and acceptable levels of ionic residue species on our two reference areas. This led us to believe that an external fluid contaminant had been introduced at some point and caused the visible white residues and dendritic growth. Looking at the board, the white residue appeared in a line like that of a drip, possibly caused by tap water, which tends to be high in chloride and sulfate.
 |
| TABLE 1: Anion ion chromatography data. |
We recommended that the customer monitor assembly processes more closely and determine the cause of the external fluid contaminant that was creating this reliability issue.
This is one of a plethora of examples we have found that is indicative of the importance of looking at boards in localized areas. If we were to use industry methods such as ROSE testing, which extract a sample from the entire board, the localized residues in this example could have been overlooked. This method would indicate that a residue problem did not exist, and that the white residues were potentially benign. Yet the residues in this case were highly corrosive and were already causing problems that would only rise in the field. Other available methods allow us to view localized areas individually to catch problems such as this before residues become visible and dendrites appear on the board.
Terry Munson is with Foresite Inc. (residues.com); tm_foresite@residues.com.
Press Releases
- PulseForge Makes Major Breakthrough Which Allows Flux-less Soldering in an Ambient Environment
- Panasonic Connect publishes case study on delivery of its surface mount technology (SMT) equipment to Dixon Technologies, one of India’s largest EMS companies
- CE3S and Desco Industries Announce May 2026 ANSI/ESD S20.20-2021 Certification Webinar Series
- Kurtz Ersa Goes Semiconductor: Expanding Competence in Microelectronics & Advanced Packaging







