Current Issue
Protecting Silicon with Silicone
New systems are more robust, faster curing and require less energy than conventional chemistries.
With each passing day, use of silicone rubber is becoming more and more pervasive; products made from silicone are showing up in hospitals, cars, the kitchen and bath, even golf bags. Flexible bakeware and cooking utensils, fuel-resistant hoses and gaskets, airbags and touch pads, shampoos, skin care products and much more are all made possible with new silicone technologies. Dr. Fredrick S. Kipping, the father of silicone chemistry, could never have envisioned the plethora of uses into which his “sticky mess” has grown. From the earliest days of commercialization, silicones have always been selected for any high-performance specialty application that requires durability and outstanding physical properties.
Silicone chemistry is a unique combination: neither purely organic (i.e., carbon-based) nor purely inorganic (i.e., silicon-based), but a molecular-level hybrid of both. The nature of the Si-O bond in the polymer backbone creates low rotation barriers and large bond energies. This inorganic polysiloxane backbone provides the foundation for building materials with superior thermal properties, environmental resistance and flexibility, even at temperatures below -70oC. The methyl groups pendant to the Si-O-Si chain provide for low surface energies, and the low rotation barrier along the backbone permits the polymer to freely orient these groups to the exposed surfaces. The hydrophobic character and soft-touch feel of silicone-based materials is a direct result of this combination.
Silicone elastomers are used as sealants, adhesives and coatings, where they are fluid-applied and cured in place, but may be also molded into a multitude of shapes and used to produce functional products in a range of applications. There are two predominate cure types: condensation-cure and addition-cure. Condensation cure products may be either single-component or dual-component, generally use tin catalysts, require moisture to react and liberate alcohol or other monomers as byproducts during cure. Addition cure materials may also be either single-component or dual-component, are either free radical cured or rely on precious metal catalysts, but perhaps the biggest distinction is that there are no byproducts generated during cure.
Certainly nearly every homeowner is familiar with single-component, condensation cure silicones, perhaps even using some to caulk the bathtub or kitchen sink: the familiar smell of vinegar as acetic acid is liberated during the condensation reaction. Single component, moisture cure, RTV silicones (room temperature vulcanizing) are valued for their ease of use, adhesion, physical properties and environmental resistance. The products are manufactured under dry conditions and stored in moisture-proof packaging. Once applied, the material draws humidity from the atmosphere and begins to cure. The cure proceeds from the outside inward, with the rate of cure determined by the amount of catalyst in the formula, but more important, by the amount of moisture available. The byproduct liberated is dependent on the leaving group of the cross-linker used in each specific formulation (Figure 1).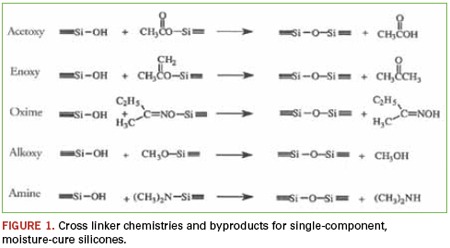
Within electronics, moisture-cure RTVs are often employed as staking compounds, where vibration dampening and stress relaxation are important. These types of staking compounds are most often shear-thinning pastes that, once applied, resist flow and may be used to build up a protective barrier around sensitive components. When fully cured, these materials become relatively soft (i.e., Shore A 25-45), durable, low modulus rubbers. Many silicone conformal coatings are also based on this type of chemistry. The advantages are ease of application, solvent-free formulations and environmental resistance of the cured films. Silicone conformal coatings are most widely used in high-temperature environments, making them the primary choice for under-the-hood automotive applications. However, because moisture from the atmosphere is needed to catalyze the cure single-component, RTVs must only be applied in thin cross-sections, typically less than 0.5", and the assembly must be racked overnight to permit slower cure speeds.
Less familiar, but no less functional, are the dual-component condensation cured silicones where the cross linker and catalyst are contained in separate packages that must be mixed prior to application. Here, the moisture is an integral part of one component, making these formulas much less sensitive to environmental conditions. Also, because the reaction is not dependent on the transport of moisture from the atmosphere, these materials are able to cure in thicker cross-sections. However, a byproduct is still generated during cure, so shrinkage is relatively high.
High consistency silicone rubber (HCR) compounds may be processed by injection molding, compression molding, and extrusion or calendaring. These are the original single-component, addition cure rubbers: typically, ready-to-use blends of silicone rubber with fillers, modifiers and vulcanizing agents that are heat-cured to form elastomeric components. The vulcanizing agents are generally peroxides that break down with heat, generating free radicals that initiate the cross-linking reactions.
Liquid silicone rubbers (LSR) are also based on addition cure chemistry. Generally, two-part pumpable materials that must be mixed and often heat-cured to form elastomers are suited for intricate designs manufactured in large, automated quantities. LSRs rely on precious metal catalysts, usually platinum, to promote cross linking. As with the condensation cure, two-component formulations, the catalyst and cross linker are contained in separate packages that must be mixed prior to application. LSRs and fully-fluorinated LSRs currently represent the fastest growing branches of the silicone family tree.
Platinum-catalyzed, addition-cure silicones may be formulated with an extremely wide range of physical properties and cure characteristics, which makes these materials very popular. One of the most exciting developments is the introduction of low viscosity, optically clear potting and encapsulating compounds. LEDs, photovoltaic subassemblies and other light-sensitive devices require radiation-resistant, non-yellowing encapsulants for protection and improved light transmission. For radiation energy to initiate any chemical changes, causing a breakdown in properties and yellowing, the polymer molecules must first absorb it. Because of the absence of any double-bonds or other ultraviolet (UV) light-absorbing groups polydimethyl siloxane-based, addition-cured silicones absorb very little ultraviolet radiation in the 300-400nm region. As mentioned, the bond strength of silicon-oxygen linkages in the polymer chain is exceptionally high, which prevents oxidation and loss of properties.
In the past, there were concerns that silicone oil migration or bleed out would contaminate sensitive electronic components. Early formulations used silicone polymers that were not stripped of low molecular weight contaminants, and electronic devices relied heavily on mechanical switches. These low molecular weight oligomers could volatize and condense on switches; because silicones are excellent dielectric materials, this could cause poor contact and device failure. Today, silicone polymers go through a much more extensive stripping process to remove and control contaminants. Device design also has improved, so the technology is not as susceptible to any potential contamination.
Heat cure, moisture cure, mixing, ovens: All are fine and effective means for curing products to generate pieces and parts for a great many applications. The real interest and some of the fascinating performance advances are in the field of UV-curing silicone rubbers, with the advantages of speed, ease of application and energy efficiencies associated with traditional UV curing to produce elastomeric materials with all of the performance enhancements of silicone.
The concept of UV curing silicones is not new. Acrylic end-capped, free-radical cure silicones were first commercialized in the early 80s. While they are technically UV cure, these dual-cure systems rely quite heavily on the traditional moisture reaction to affect full cure. The acrylated-silicones are sensitive to oxygen inhibition, require fairly significant energy input to initiate the reaction, and need prolonged exposures to complete the cure. Perhaps because they are not so robust, these materials have not enjoyed the market success of hydrocarbon-based UV cure technologies.
The thiolene “click” reaction is very powerful chemistry. Click chemistry is not one specific reaction, but more of a concept or a philosophy introduced in 2001 by K. Barry Sharpless, Ph.D. of the Scripps Research Institute.1 By definition, click reactions are simple and robust. They use only readily available starting materials, require no solvent, or chemically benign solvents (e.g., water), and proceed to high conversions. Applying these concepts to polymerization reactions yields materials that are extremely dynamic. Thiolene chemistry is the reaction between thiol groups and vinyl functionality. If the molecules involved contain higher functionality (i.e., F(x) >/= 2), then the reaction will produce polymeric materials. The process is normally photoinitiated and proceeds via a very rapid step-growth mechanism; an idealized outline of the initiation – propagation – chain-transfer reactions is shown in Figure 2.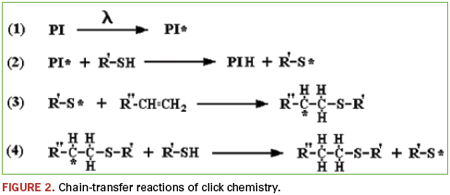
In addition to the polymerization reactions discussed earlier, the thiolene reaction may also be used as a cross-linking mechanism. High molecular weight, vinyl functional polymers may be cured with short-chain, or monomeric, multi-functional thiolene curing agents via the same photoinitiated reaction. It is essentially an addition reaction, so no byproducts are produced, and shrinkage is low. There are hundreds of vinyl and thiol combinations. Vinyl functional polydimethylsiloxanes are abundant and readily available, and there is a fairly wide selection of short-chain mercapto-functional polysiloxanes. This combination may be employed to produce UV-cured silicones.2,3 These systems were originally investigated as possible release agents for paper coatings.
This chemistry has been extended to include a secondary moisture reaction for curing in shadow areas and to further increase cure strength, adhesion and system dynamics.4 Clear materials based on this new system are much more robust and cure to greater depths with much lower energy requirements than conventional acrylated silicones. This translates to increased processing speeds and faster turnaround. Essential for protecting electronic circuitry and significantly extending the service life of printed circuit boards, conformal coatings are an integral component of the entire sub-assembly. Eliminating moisture and contamination is a key element for protecting sensitive electronics. Silicone conformal coatings provide an effective barrier, even under the most severe service conditions. Now UV-cured conformal coatings that offer all the enhanced performance characteristics of conventional silicone-based materials, but with higher processing speeds, are available.
Perhaps more important, these new UV-cure, silicone resins may be filled, which means pigmented and even electrically conductive UV-cure silicone products are now possible. Flexible and printed electronics (FPE) incorporate several new technologies and emerging processes and materials across a variety of applications. FPE may be twisted, bent or shaped without damage, enabling endless innovation and unlimited possibilities.
When an application requires performance, durability and physical properties, a silicone-based product is the superior choice. No other chemistry provides the environmental resistance and performance at thermal extremes. While it is true that silicones are critical for applications under the most severe and harsh conditions, it is also true that the enhanced adhesion, increased flexibility and UV and moisture resistance are important for many less-demanding applications.
References
1. H. C. Kolb, M. G. Finn and K. B. Sharpless, “Click Chemistry: Diverse Chemical Function from a Few Good Reactions,” Angewandte Chemie International Edition 40 (11): 2004–2021, 2001.
2. Lee, et al., U.S. Patents Nos. 4,946,874 (1990) and 5,124,212 (1992), Dow Corning Corp.
3. Viventi, U.S. Patent No. 3,816,282 (1974), General Electric Co.
4. Chambers, et al., U.S. Patent No. 7,105,584 (2006), Novagard Solutions.
Brian Chambers is director of technology, Novagard Solutions (novagard.com); bchambers@novagard.net.
Press Releases
- PulseForge Makes Major Breakthrough Which Allows Flux-less Soldering in an Ambient Environment
- Panasonic Connect publishes case study on delivery of its surface mount technology (SMT) equipment to Dixon Technologies, one of India’s largest EMS companies
- CE3S and Desco Industries Announce May 2026 ANSI/ESD S20.20-2021 Certification Webinar Series
- Kurtz Ersa Goes Semiconductor: Expanding Competence in Microelectronics & Advanced Packaging







