Field Performance of pH neutral Cleaning Agents
Part one of a series on material compatibility and field cleaning performance of alternatives to alkaline cleaning agents.
At SMTA International 2010, a study titled “Benchmark Study: pH neutral vs. Alkaline Cleaning Agents” was presented.1 That study detailed a comparative analysis of the results of short- and long-term effects of pH neutral and alkaline cleaning agents on sensitive materials such as anodized, alodine and iridite coatings, electroless nickel plating, as well as aluminum and copper. It also compared the cleaning effectiveness of each on the substrate surface and undercomponent utilizing spray-in-air inline cleaning equipment. Based on the results of the 2010 study, pH neutral cleaning agents held the promise of offering superior material compatibility, comparable if not improved cleaning performance and lower concentrations compared to alkaline alternatives for cleaning no-clean, RMA and OA flux residues. This article presents three customer case studies whereby the specific customer explored the option of employing a pH neutral cleaning agent due to issues with alkaline cleaning agents and/or DI water with their cleaning processes.
Case Study Review – Customer A. Company A is a global OEM designing and producing electronics assemblies for military, aerospace, automotive, industrial and medical applications. A new customer was acquired, and the plant in question was tasked with manufacturing the newly designed PCBs.
Within its current SMT process, this plant used an aqueous-based inline spray-in-air cleaning process and an alkaline cleaning agent. Although this process produced satisfactory results, material compatibility issues arose with one particular substrate that was manufactured with Pb-free solder paste and liquid flux.
This PCB was double-sided and populated with capacitors, microprocessors and various-sized resistors and connectors. The substrate included pluggable press-fit cages or shields with a NiAg coating (FIGURES 1 and 2).

Figure 1. PCB side 1.
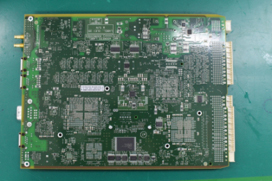
Figure 2. PCB side 2.
It should be noted it was critical to maintain the integrity of the shield material throughout the manufacturing process. As doubled-sided substrates, they were passed through reflow and wave consecutively, and then cleaned employing the current cleaning process. As this system had been satisfactorily used for years, plant personnel planned to use this system for cleaning the new substrate.
Utilizing the alkaline cleaning agent at 15% concentration and a belt speed of 0.5'/min., two passes through the cleaner were required due to the resulting burned-in fluxes from multiple heat cycles. However, as the substrates were cleaned, the surface of the shields became degraded. Thus, a new cleaning process was required. Furthermore, as the initial cleaning trials progressed, a second material compatibility issue developed as a result of an additional assembly step. A third party-sourced subassembly with a polyurethane conformal coating had to be added to the main substrate prior to cleaning. As the OEM attempted multiple wash cycles to achieve the desired cleanliness level, the added wash time caused the conformal coating to peel.
The customer partnered with the company and developed a two-part design of experiments (DoE) to resolve both material compatibility issues:
Part 1: Utilizing its current cleaning system, identify an alternate aqueous-based cleaning agent, and utilizing the customers current cleaner, develop the process parameters to achieve the desired cleaning results without degrading shield coating.
Part 2: Utilizing the cleaning process parameters developed in Part 1, assess the compatibility of the selected cleaning agent with conformal coating materials, recommending an optimum solution.
Part 1: DoE. The DoE was based on using the current inline cleaning system. Based on the material compatibility issues identified, a pH neutral cleaning agent was selected for the initial trials.
Preliminary cleaning trials were conducted in the customer plant using the pH neutral cleaning agent. Optimum parameters were developed, as detailed in TABLE 1. Using these conditions, three production boards were cleaned and sent to the Zestron technical center in Manassas, VA, for a cleanliness assessment. The boards were analyzed through visual inspection and ion chromatography. The inspected boards were returned to the customer for functional tests.
Table 1. Preliminary Cleaning Trial Parameters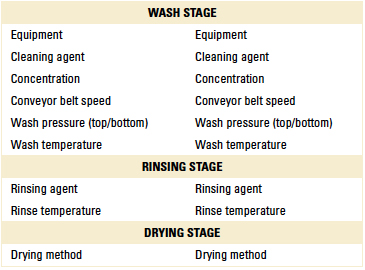
Visual inspection results. Visual inspection was conducted following IPC-A-610E. All of the boards were found to be fully cleaned (FIGURES 3 to 6). Shield surface was unaffected (FIGURES 7 and 8).
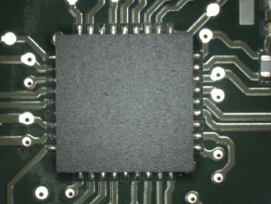
Figure 3. Bottom-side visual inspection.
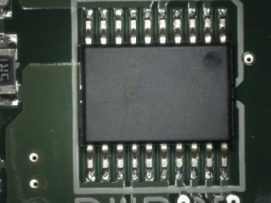
Figure 4. Bottom-side visual inspection.
Figure 5. Top-side visual inspection.
Figure 6. Top-side visual inspection.

Figure 7. Top-side shield surface.

Figure 8. Top-side shield surface.
As indicated through visual inspection, the substrate top and bottom surfaces were fully cleaned, and the surfaces of the shields were left unaffected; therefore, they were found to be fully compatible with the pH neutral cleaning agent.
Ion chromatography results. Ion chromatography was conducted on all three boards per IPC-TM-650, Method 2.3.28.2. Results are detailed in TABLE 2.
Table 2. Part 1 Ion Chromatography Results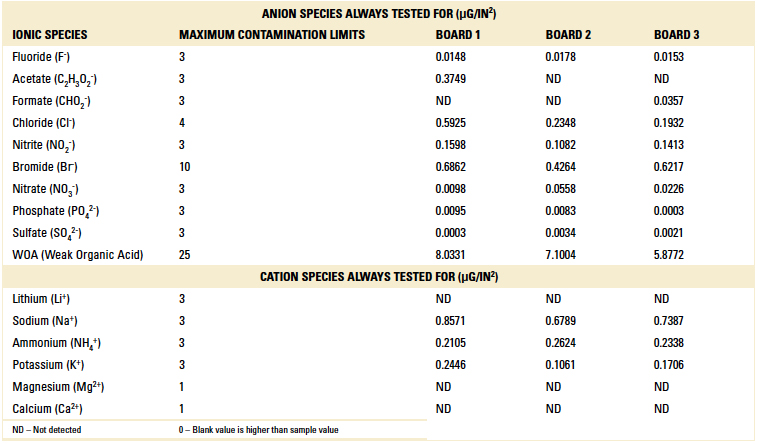
All boards passed, further validating the cleanliness level desired was achieved with the pH neutral cleaning process. The boards were returned to the customer and found to be fully functional.
Part 2: DoE. Following Part 1 trials and cleanliness assessments, the customer was satisfied that the pH neutral cleaning process could result in achieving the desired cleanliness levels for the new substrates and is fully compatible with all materials used. Within Part 2 of the DoE, they assessed the compatibility of the optimized cleaning process with conformal coating materials.
Three coating types were compared for the initial compatibility analysis. These were parylene, acrylic and polyurethane. Based on internal tests conducted at the company R&D Center, parylene coating was found to be most compatible with the pH neutral cleaning agent. Thus, the next step was to coat substrates with the parylene and evaluate compatibility within the customer’s cleaning process using the operating conditions identified in Part 1.
In this case, the substrate used was an unpopulated company test vehicle (FIGURE 9). Three test boards were sent to the coating supplier for the parylene application. Each board was coated with a 33µm layer of parylene-based conformal coating material. The boards were returned to the company technical center where they were subjected to a cleaning process using an inline cleaner, similar to the one used by the customer, and operated with the same parameters as defined in Part 1. However, in this case, the boards were passed through the cleaner twice. Following the cleaning process, they were visually inspected under high magnification following the IPC-TM-610E.
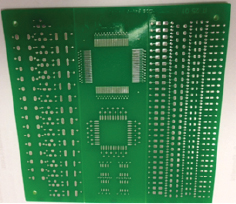
Figure 9. Part 2 DoE test vehicle.
Parylene coating visual inspection results. Based on the visual inspection results of the coated test vehicle, following the first and second pass through the inline cleaner, the coating was found to be intact (FIGURES 10 to 13).
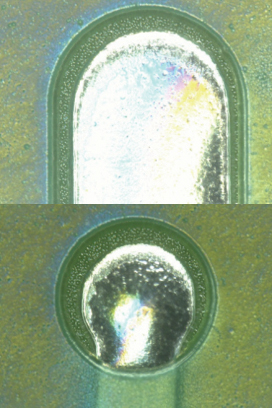
Figure 10. Parylene coating before cleaning.
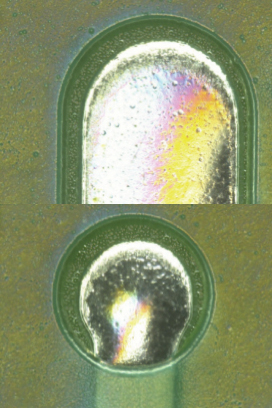
Figure 11. Parylene coating after one pass.
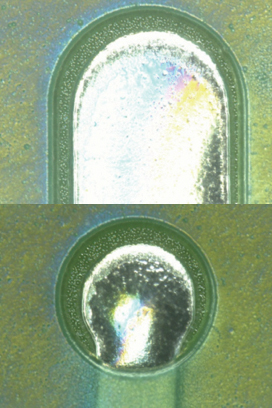
Figure 12. Parylene coating before second pass.
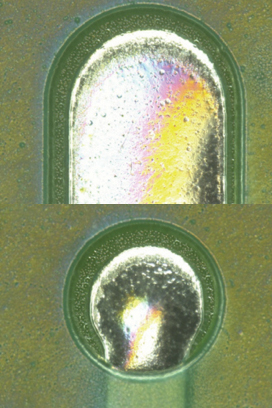
Figure 13. Parylene coating after second pass.
Conclusions. The pH neutral cleaning process identified through the Part 1 cleaning trials accomplished the following:
- Fully cleaned the double-sided substrates assembled with Pb-free solder paste and flux using the customer’s current spray-in-air inline cleaner, and optimized for the pH neutral cleaning agent.
- Exhibited excellent material compatibility with the nickel silver shield coating.
- Exhibited excellent material compatibility with the parylene conformal coating.
References
1. Dr. Harald Wack, “Benchmark Study: pH-Neutral vs. Alkaline Cleaning Agents,” Proceedings of SMTA International, October 2010.
Ed.: This article was originally published as “pH neutral Cleaning Agents – Market Expectation and Field Performance” in the IPC Apex Expo Proceedings and is republished here with the authors’ permission. Part 2 can be read here.
, is application technology manager, , is senior applications engineer, , is application engineer, and is a former application engineer at Zestron Americas (zestronusa.com); umut.tosun@zestronusa.com.




